
全部
▼
搜索
熱搜:
位置:中冶有色 >
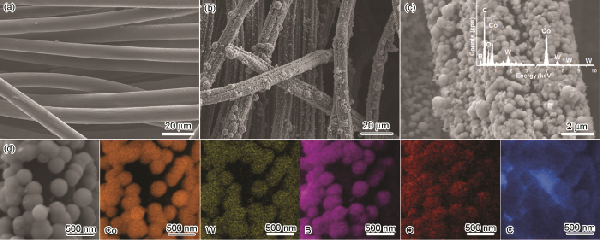
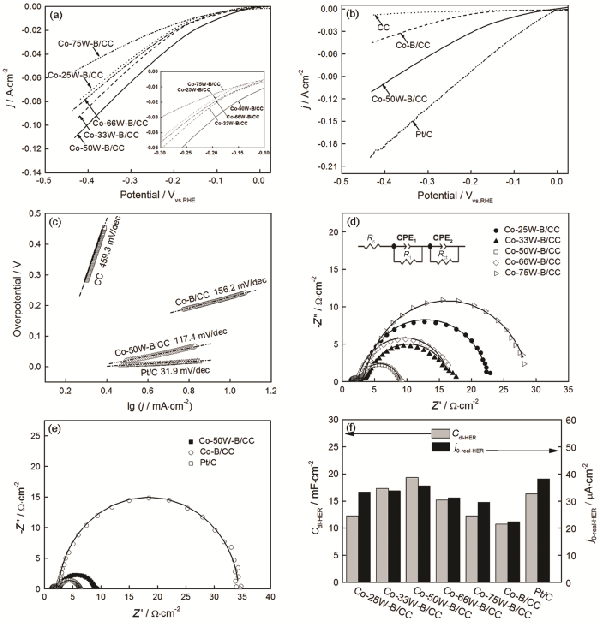
> 非晶Co-W-B/碳布復(fù)合電極材料的制備及其電解水催化性能
 924
編輯:中冶有色技術(shù)網(wǎng)
來源:施嘉倫,盛敏奇,吳瓊,呂凡
924
編輯:中冶有色技術(shù)網(wǎng)
來源:施嘉倫,盛敏奇,吳瓊,呂凡




 分享 0
分享 0
 舉報(bào) 0
舉報(bào) 0
 收藏 0
收藏 0
 反對(duì) 0
反對(duì) 0
 點(diǎn)贊 0
點(diǎn)贊 0

 中冶有色技術(shù)平臺(tái)
中冶有色技術(shù)平臺(tái) 2024年12月27日 ~ 29日
2024年12月27日 ~ 29日  2025年01月03日 ~ 05日
2025年01月03日 ~ 05日  2025年01月03日 ~ 05日
2025年01月03日 ~ 05日  2025年01月03日 ~ 05日
2025年01月03日 ~ 05日  2025年03月25日 ~ 27日
2025年03月25日 ~ 27日 
