
全部
▼
搜索
熱搜:
 798
編輯:中冶有色技術網
來源:劉欣怡,王仕發(fā),余先倫,唐盛楠,房雷鳴,雷力
798
編輯:中冶有色技術網
來源:劉欣怡,王仕發(fā),余先倫,唐盛楠,房雷鳴,雷力
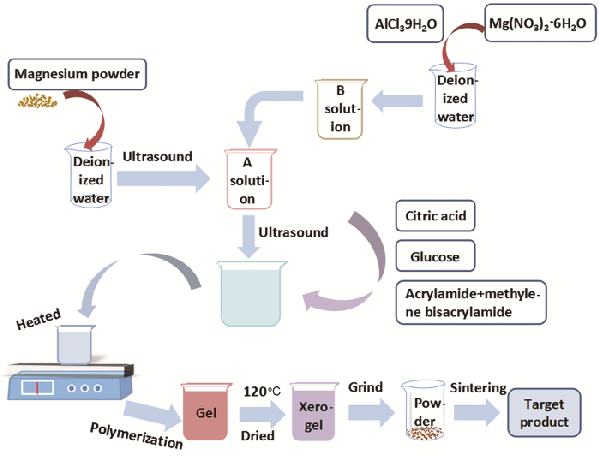
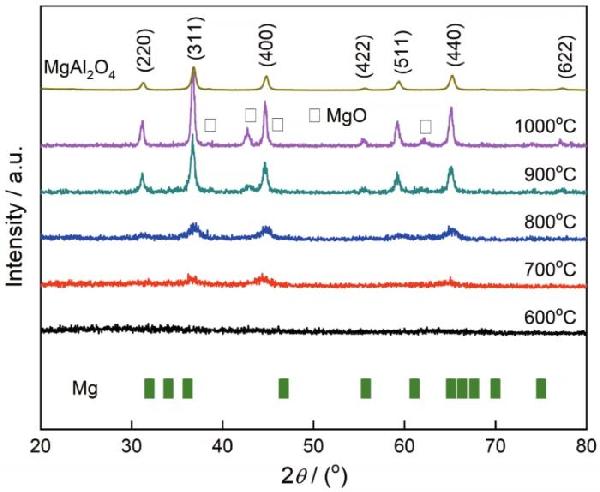
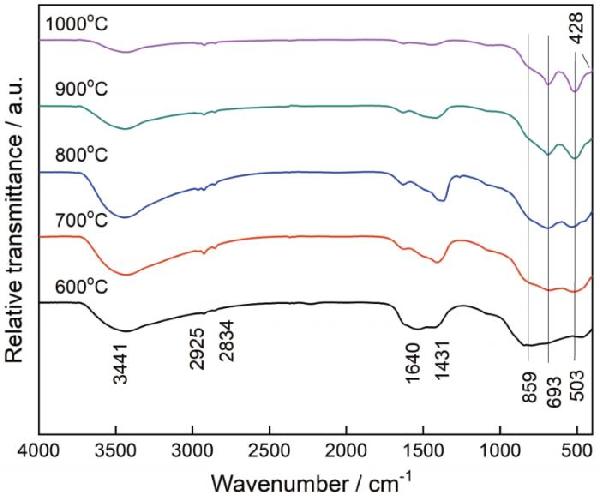


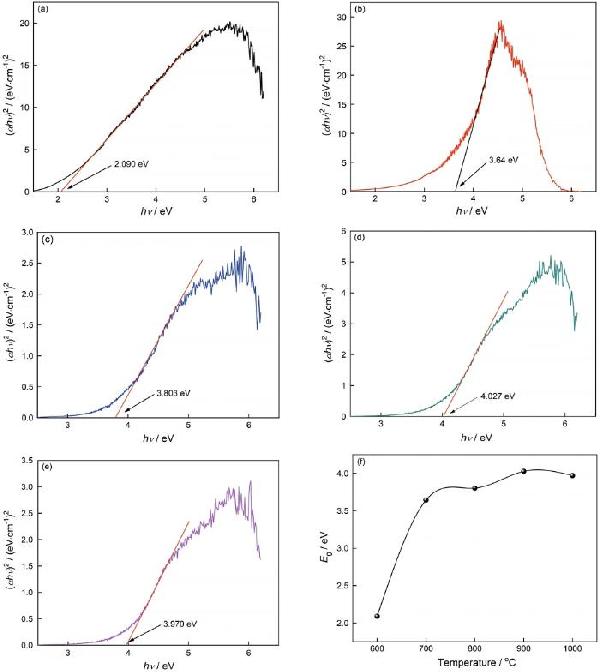
| Sample | Color coordinates | Eg /eV | |||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | c* | Ho | ECIE* | ||
| 600 | 55.878 | 5.226 | 13.080 | 14.085 | 68.221 | 57.626 | 2.090 |
| 700 | 70.892 | 0.345 | 10.249 | 10.296 | 88.072 | 71.636 | 3.643 |
| 800 | 98.318 | -0.497 | 4.307 | 4.336 | -83.418 | 98.414 | 3.803 |
| 900 | 98.049 | -0.531 | 3.055 | 3.101 | -80.140 | 98.098 | 4.027 |
| 1000 | 97.652 | -0.161 | 2.391 | 2.396 | -86.148 | 97.681 | 3.970 |





 分享 0
分享 0
 舉報 0
舉報 0
 收藏 0
收藏 0
 反對 0
反對 0
 點贊 0
點贊 0

